- Product
- Industrial Zirconia
- Rare Earth Products
- Alumina
- Carbide
- Nitride
- Ceramic Products
- Graphite Products
- Nano Materials
- Spherical Spray Material
- High Purity Metal Powder
- Hot Selling Advanced Materials
01
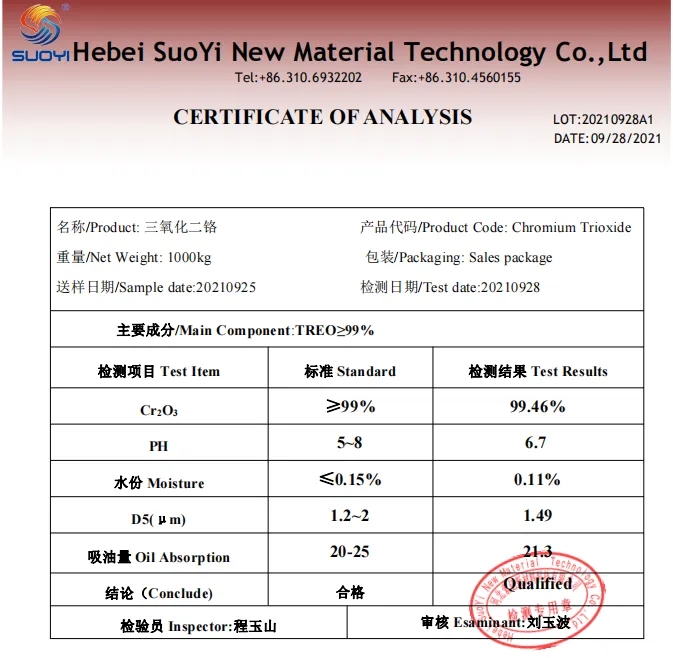
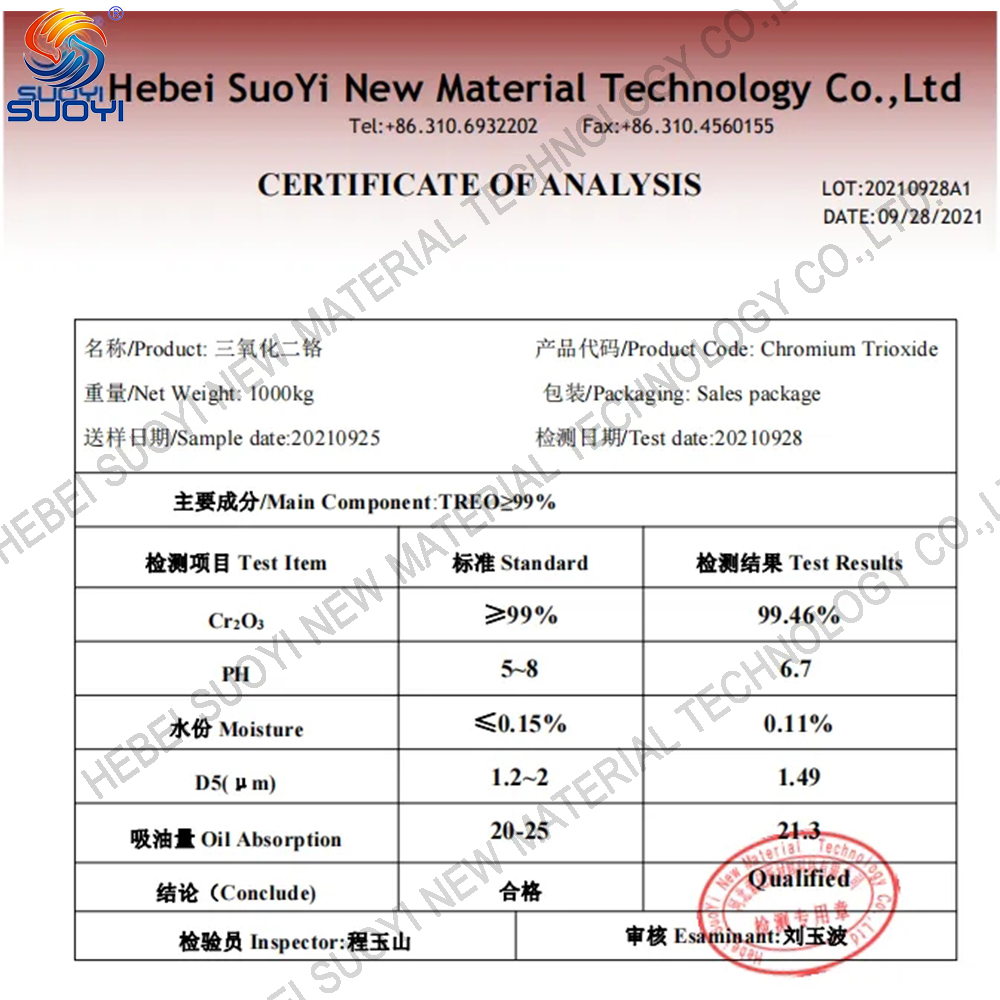
Suoyi Factory Spherical chromium oxide CAS 1308-38-9
|
Product Name |
Spherical Chromium(III) oxide |
|
CAS |
1308-38-9 |
|
Purity |
99%min |
|
Appearance |
Green powder |
|
MOQ |
10g |
|
Melting point |
2435 °C |
|
Boiling point |
4000 °C |
|
Density |
5.21 |
|
Refractive index |
2.551 |
|
Usage |
organic synthesis intermediates |
|
Storage conditions |
Room Temperature |
|
Certificate |
ISO/COA/MSDS/TDS |
Chromium oxide (Cr2O3) is an inorganic compound
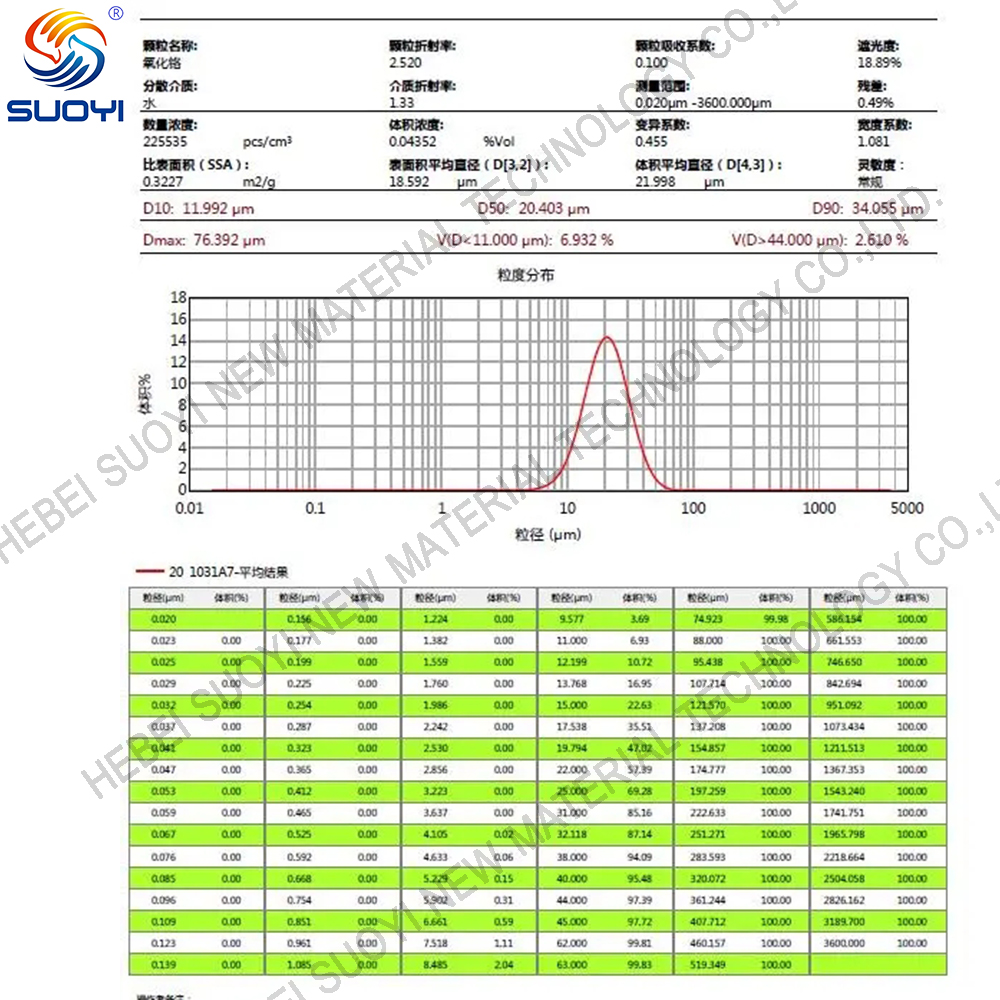
1. Appearance: Chromium oxide is a green crystalline particle or powder.
2. Melting point and boiling point: The melting point of chromium oxide is about 2435 ° C, and the boiling point exceeds 4000 °C.
3. Density: The density of chromium oxide is approximately 5.22 g/cm ³。
4. Chemical properties: Chromium oxide is a highly stable compound that is insoluble in water and most solvents. It has high resistance to acid and alkali. In addition, chromium oxide has good wear resistance and high temperature resistance.
Chromium oxide has multiple uses
1. Pigment: Due to its bright green color, it is commonly used as a green pigment in industries such as ceramics, glass, coatings, and plastics.
2. Corrosion inhibitor: Chromium oxide can be used as a corrosion inhibitor for steel, alloys, and other metals to extend their lifespan.
3. Catalyst: Chromium oxide can be used as a catalyst in chemical reactions, such as ammonia oxidation reactions.
4. Electronic materials: Due to its excellent electronic properties, chromium oxide is widely used in electronic devices, magnetic materials, and optical coatings.
There are usually several methods for producing chromium oxide:
1. Thermal decomposition method: Heat and decompose the chromium salt solution to generate chromium oxide.
2. Oxidation method: Use oxygen to oxidize metal chromium into chromium oxide.
3. Hydrothermal method: Chromium salt reacts with water to form crystals under high temperature and pressure conditions.
When using chromium oxide, the following safety information should be noted:
1. Chromium oxide is a toxic substance, avoid inhaling particles and exposing it to the skin.
2. During operation, appropriate personal protective equipment such as gloves and protective goggles should be worn.
3. Avoid mixing with acidic or flammable substances to avoid the generation of toxic gases or fires.
4. When storing, it should be sealed to avoid contact with other chemicals.
01









 Mr. Perry Wu International Sales Director
Mr. Perry Wu International Sales Director